Return from ECTRIMS 2024
We are thrilled to share the key moments and insights from the 40th ECTRIMS Congress held from Wednesday, 18 September to Friday, 20 September in Copenhagen.
This page is your gateway to:
- New McDonald Criteria: Discover the latest updates and implications in MS diagnosis.
- Congress Highlights: Watch exclusive video clips featuring Belgian experts discussing groundbreaking topics.
- Novartis Abstracts and Scientific Posters about Kesimpta: Review long-term 6 years Ofatumumab data and its impact on treatment.
- Reach out your Novartis Partners: Connect with our partners for further information and collaboration opportunities.
Thank you for joining us and continuing the journey of innovation and discovery.
Summary
- 2024 McDonald criteria: updates in MS diagnosis
- Congress Highlights: video clips
- Novartis abstracts and scientific posters about Kesimpta
- Reach out your Novartis partner
2024 McDonald Criteria: Updates in Multiple Sclerosis Diagnosis
2024 McDonald Criteria: Updates in Multiple Sclerosis Diagnosis
The revised McDonald criteria were eagerly anticipated within the MS community, and are set to significantly impact the way MS is diagnosed and managed.
They were presented by Prof Xavier Montalban at the ECTRIMS 2024 congress and are being discussed by Prof Dive and Prof Laureys in the links below.
Some of the important changes include:
- New MRI markers – the central vein sign (CVS) and paramagnetic rim lesions (PRL) – have been added to the criteria
- The optic nerve may serve as a fifth anatomical location to demonstrate dissemination in space (DIS) and can be shown by MRI, visual evoked potentials (VES) or optical coherence tomography (OCT)
- Diagnosis of MS will be allowed in some patients who would be classified as having radiologically isolated syndrome (RIS) (= presence of MRI brain lesions but no overt clinical symptoms)
- Kappa free light chains can be used as alternative to the presence of oligoclonal bands
References:
1 https://ectrims.eu/videos/prof-xavier-montalban-on-the-revised-mcdonald-criteria-a-new-era-in-ms-diagnosis-management/
2 ECTRIMS 2024 congress: Key Takeaways by Prof Dive on 18 sept 2024
3 ECTRIMS 2024 congress: Key Takeaways by Prof Dive on 20 sept 2024
Congress Highlights: video clips
Check out our three video clips featuring Prof. D Dive (CHU Liège), Dr. T. Reynders (UZA), and Prof. G. Laureys (UZ Gent) as they share their insights on various scientific topics from Ectrims 24.
Ectrims 24 Day1: New diagnostic criteria with Pr. D. Dive (CHU Liège)
Ectrims 24 Day 2: Exploring MS phenotypes and advances in NMOSD with Dr. T. Reynders (UZA)
Ectrims 24 Day 3: key take away messages from Pr. G. Laureys (UZ Gent)
Novartis abstracts and scientific posters about Kesimpta
This section contains our main scientific posters along with brief summaries that were presented at the congress
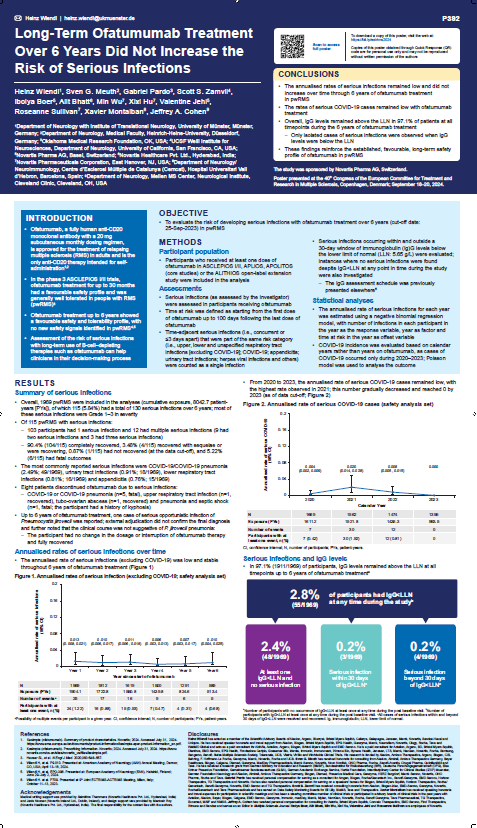
Abstracts P392:
Long-Term Ofatumumab Treatment Over 6 Years Did Not Increase the Risk of Serious Infections
Summary:
The 6-year study on Kesimpta® demonstrates a consistently low and stable annualized rate of serious infections, including COVID-19, with most patients (97.1%) maintaining IgG levels above the lower limit of normal. This reinforces the favorable long-term safety profile of Kesimpta®, making it a reliable treatment option for relapsing multiple sclerosis
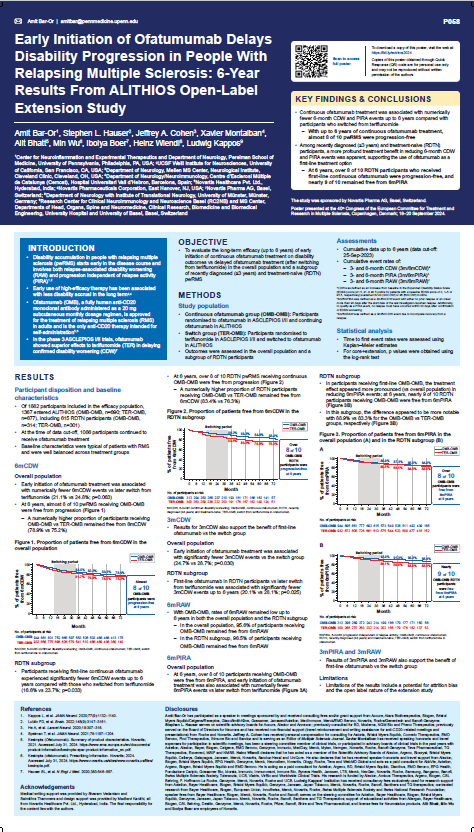
Abstract P058:
Early Initiation of Ofatumumab Delays Disability Progression in People With Relapsing Multiple Sclerosis: 6-Year Results From ALITHIOS Open-Label Extension Study
Summary:
The 6-year ALITHIOS study shows that early initiation of Kesimpta® treatment results in fewer disability worsening events compared to those who switched later from teriflunomide. Specifically, 78.9% of patients who started Kesimpta® early were free from 6-month confirmed disability worsening (6mCDW) at 6 years. This reinforces the benefits of starting Kesimpta® treatment earlier for patients with relapsing multiple sclerosis.
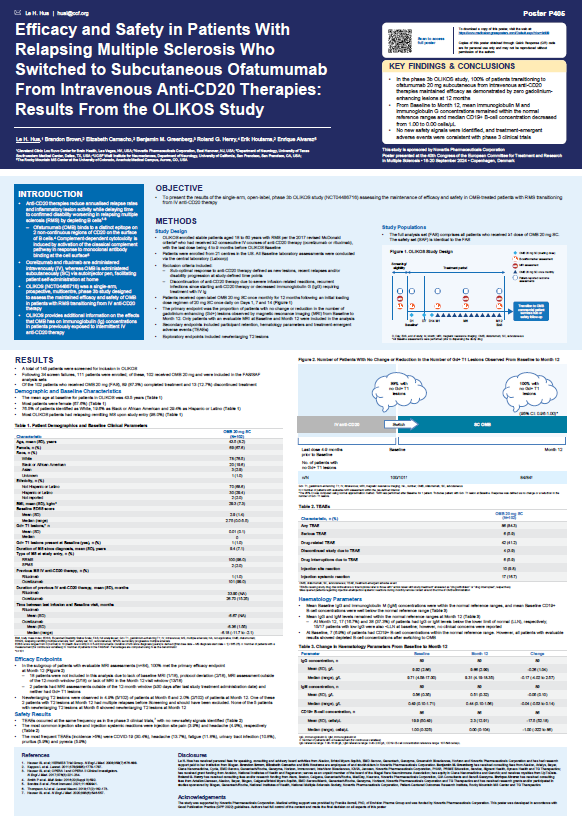
Abstract P405:
Efficacy and Safety in Patients With Relapsing Multiple Sclerosis Who Switched to Subcutaneous Ofatumumab From Intravenous Anti-CD20 Therapies: Results From the OLIKOS Study
Summary:
The phase IIIb OLIKOS study demonstrates that 100% of patients switching to Kesimpta® from intravenous anti-CD20 therapies maintained efficacy, with zero T1 lesions at 12 months. Additionally, mean immunoglobulin M and immunoglobulin G concentrations remained within normal reference ranges, and no new safety signals were identified. This is reinforcing the favorable safety profile and the efficacy of Kesimpta® after switching from a HET.
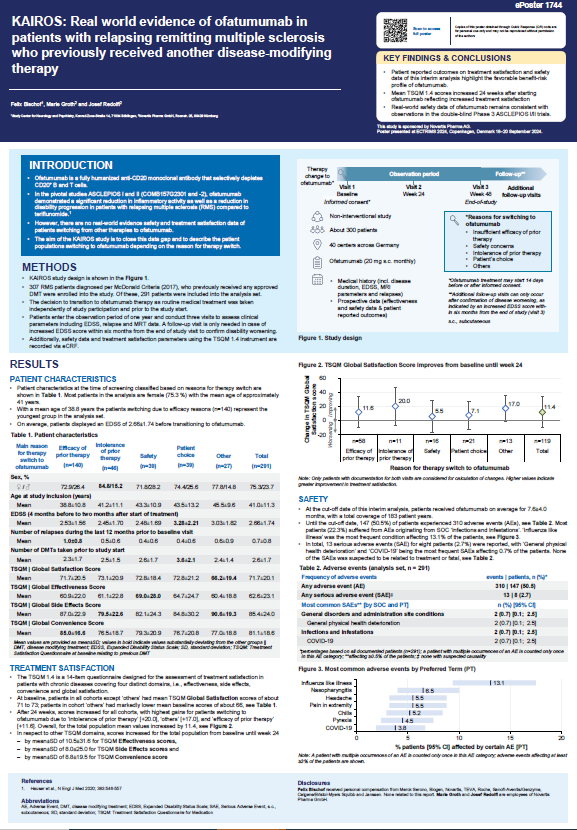
Abstracts P1744:
KAIROS: Real world evidence of ofatumumab in patients with relapsing remitting multiple sclerosis who previously received another disease-modifying therapy
Summary:
The study indicates that patients mainly switched to Kesimpta® due to inadequate effectiveness, safety issues, and intolerance of their previous therapy. Furthermore, treatment satisfaction increased 24 weeks after starting Kesimpta®, with safety data aligning with the Phase 3 ASCLEPIOS I/II trials. This highlights Kesimpta® effectiveness and safety for those transitioning from other treatments.
Reach out your Novartis partner

If you would like further details about Kesimpta and its long-term data for your MS patients, please complete the form, and your contact at Novartis will get in touch with you shortly.
Click below and fill the form to reach our team and set up an appointment
Neuroscience Team fast access | my.novartis.be

1.KESIMPTA SmPC
2.Wiendl H, et al. Longer-term Safety and Efficacy of Ofatumumab in People With Relapsing Multiple Sclerosis for Up to 6 Years. Poster presentation at the American Academy of Neurology (AAN) 2024 Annual Meeting; April 13-18, 2024; Denver, CO,USA.
Related Content
Spotlight on MS – Latest insights
Discover 9 movies from 9 international MS experts sharing their insights on the future of MS treatment.
Congress abstracts & publications
Find here abstracts published at the latest congresses and scientific publications.
Training videos & webinars
Discover short capsules videos around MS and recorded webinars from international and Belgian KOL’s.